We once received an urgent call from a shipyard in Dubai. Their standard brass fittings had corroded into a powdery mess within months, halting a critical project. This failure taught us a crucial lesson about brass and saltwater.
Yes, brass elbows can be used in marine and coastal environments, but only if they are made from specific, corrosion-resistant alloys and often with additional protective coatings. Standard brass will corrode quickly in saltwater, leading to leaks and failures. Success depends entirely on choosing the right material and maintenance plan for the harsh conditions.
The ocean air and water create a perfect storm for metal corrosion. Let’s navigate the specifics of making brass work where it matters most.
What Specific Brass Alloys Offer Superior Resistance to Saltwater Corrosion?
Choosing the wrong brass alloy is the most common and costly mistake. Not all brass is created equal for the sea.
Specific brass alloys with high copper content and added inhibitors like tin, aluminum, or arsenic offer superior saltwater resistance. Key marine-grade alloys include Naval Brass (C46400), Aluminum Brass (C68700), and Arsenical Admiralty Brass (C44300). These alloys significantly slow down the primary corrosion mechanism called dezincification.

Understanding the Alloy Composition
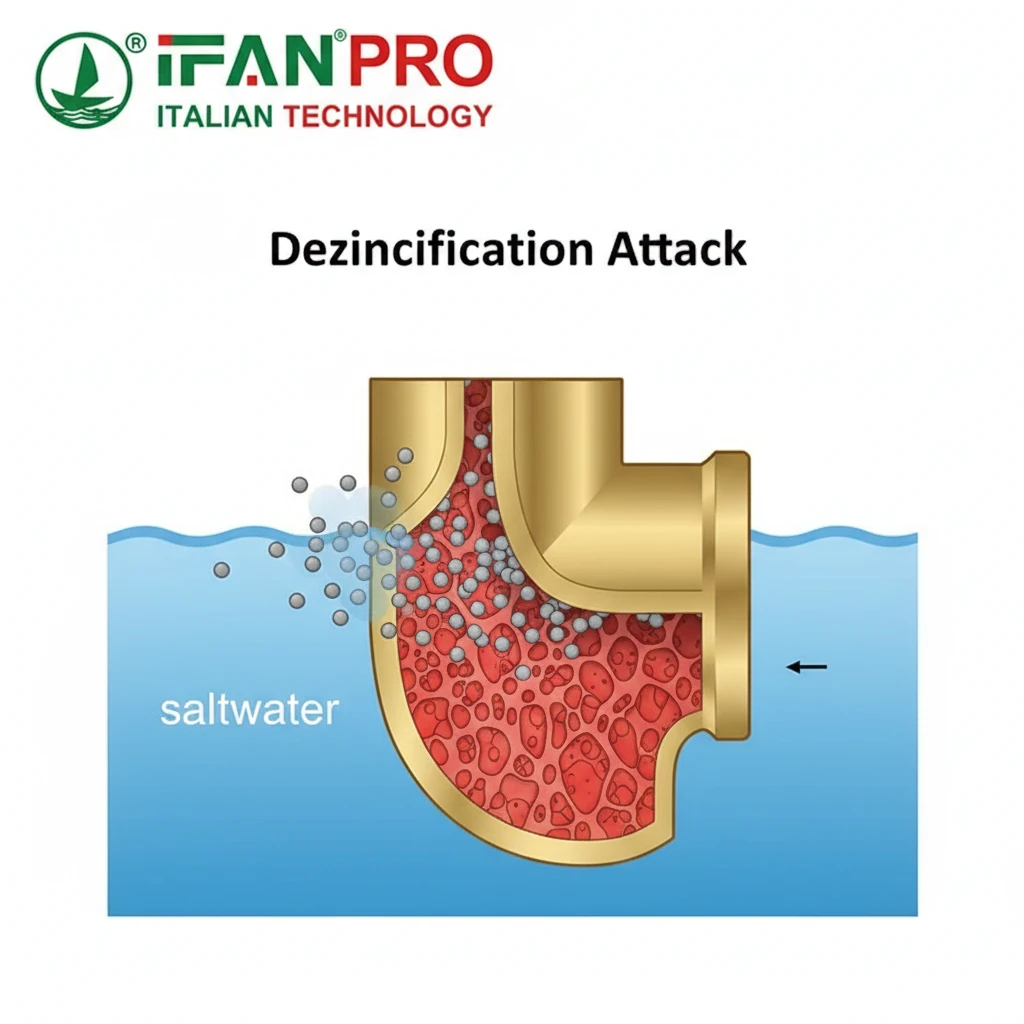
Brass is an alloy of copper and zinc. The problem in seawater is the zinc. Saltwater aggressively attacks and leaches zinc out of the alloy, leaving behind a weak, porous, and copper-rich structure that can easily crumble. This process is dezincification.
To fight this, marine-grade brasses change the formula. They reduce the zinc content and add other metals that act like bodyguards for the alloy.
- Naval Brass (C46400): This is the classic marine workhorse. It has about 60% copper, 39% zinc, and a crucial 1% tin. The tin forms a stable, protective film on the surface that resists saltwater corrosion. It’s strong and very reliable for fittings, pumps, and propeller shafts.
- Aluminum Brass (C68700): This alloy contains around 77% copper, 21% zinc, and 2% aluminum. The aluminum reacts with seawater to form an extremely hard, protective aluminum oxide layer on the surface. This makes it excellent for condenser tubes and heat exchangers where water flows quickly.
- Arsenical Admiralty Brass (C44300): This alloy has about 71% copper, 28% zinc, 1% tin, and a tiny amount of arsenic (0.02-0.06%). The arsenic acts as a potent inhibitor, dramatically slowing down the dezincification process from within the metal’s grain structure.
Choosing the Right Alloy for the Job
The best choice depends on your specific application. Consider water speed, temperature, and the presence of sand or debris.
| Alloy Name (UNS Number) | Key Composition | Best Use in Marine Environments | Main Advantage |
|---|---|---|---|
| Naval Brass (C46400) | CuZn39Sn1 | Valves, fittings, fasteners, propeller shafts | Excellent all-around strength and corrosion resistance. |
| Aluminum Brass (C68700) | CuZn21Al2 | Condenser tubes, heat exchangers, high-flow piping | Forms a super-hard protective layer against fast-moving water. |
| Arsenical Admiralty Brass (C44300) | CuZn28Sn1As | General piping, low to medium-flow seawater systems | Superior internal protection against dezincification in slow/stagnant water. |
In short, you must specify the alloy by its common name or UNS number. Never just order “brass fittings” for a marine project.
How Does Dezincification Risk Increase in Marine Atmospheres?
Dezincification is the silent killer of brass in saltwater. The marine environment makes this process much faster and more severe.
Dezincification risk increases dramatically in marine atmospheres because salt (sodium chloride) acts as a powerful electrolyte. It creates highly conductive water that accelerates the electrochemical reaction which leaches zinc from the brass. Warm water temperatures, low water flow, and high oxygen content—all common at sea—further speed up this destructive process.
The Chemistry of the Problem
To understand the risk, think of a battery. A battery needs two different metals and an electrolyte (like saltwater) to create a current. In a piece of brass, the copper and zinc are the two different metals, and they are microscopically close together. Saltwater is the perfect electrolyte.
This creates thousands of tiny batteries on the surface of the fitting. In this reaction, the zinc “sacrifices” itself and dissolves into the water. What’s left behind is a spongy, brittle copper structure that has no mechanical strength. A fitting can look intact on the outside but be completely weak and porous inside, leading to sudden failure.
Factors That Turn Up the Risk
The marine environment adds several factors that turn this process from slow to rapid:
- The Salt Itself: More salt means more conductivity. Ocean water is a far stronger electrolyte than fresh water.
- Warm Temperatures: Chemical reactions, including corrosion, roughly double in speed for every 10°C (18°F) increase in temperature. Seawater in tropical regions or engine cooling systems is especially aggressive.
- Stagnant or Low-Flow Water: When water sits still, the dissolved zinc ions are not carried away. This allows a concentrated layer to build up right at the metal surface, which actually drives the reaction faster. Stagnant water in a pipe or a fitting is a dezincification hotspot.
- Oxygen-Rich Water: Splashing waves, spray, and aerated water introduce more oxygen. Oxygen is a key player in the corrosion reaction; more oxygen means a faster reaction.
Visual Signs and Proactive Checks
You can often spot dezincification before a fitting fails completely. Look for these signs:
- Color Change: The fitting turns from a yellow-gold brass color to a reddish, copper-only color.
- Chalky or Powdery Residue: You might see white powder (zinc salts) around the fitting.
- Weak Structure: If you scratch a suspect area with a knife, the metal may crumble easily instead of showing a shiny, metallic scratch.
For critical applications, regular inspection is non-negotiable. The marine atmosphere doesn’t forgive oversight.
Are Protective Coatings or Plating Required for Long-Term Marine Service?
Even with the right alloy, adding an extra layer of defense is often a smart investment for long-term peace of mind.
For long-term marine service on critical components, protective coatings or plating are highly recommended and often required. They provide a physical barrier against salt and oxygen, buying significant time even if the coating gets scratched. For less critical or submerged applications, a high-integrity marine-grade alloy may be sufficient on its own.

The Role of Coatings as a Barrier
Think of a coating as a raincoat for your brass fitting. The best marine-grade brass is like wearing a quick-dry, water-resistant shirt—it’s good, but a raincoat on top is better in a storm.
The primary job of a coating is to keep the electrolyte (saltwater) from touching the metal. No contact means no electrochemical corrosion cell can form. This is especially important for parts exposed to salt spray in the “splash zone,” which is one of the most corrosive areas on a boat or coastal structure.
Common and Effective Coating Options
Several types of coatings work well for brass in marine environments:
- Electroplating: This involves electrically bonding a thin layer of another metal onto the brass.
- Nickel Plating: Provides a very hard, shiny, and corrosion-resistant barrier. It’s common for decorative and functional marine hardware.
- Chromium Plating (“Chrome”): Often plated over nickel. It adds a bright finish and extra corrosion resistance, but can show pitting if the underlying nickel layer is compromised.
- Hot-Dip Galvanizing: This involves dipping the brass fitting into molten zinc. It creates a thick, rugged coating that offers sacrificial protection. The zinc coating will corrode first, protecting the brass underneath. This is excellent for structural components.
- Polymer/Powder Coatings: These are layers of epoxy, nylon, or other plastics sprayed and baked onto the metal. They offer excellent color options and a thick barrier. Quality depends heavily on surface preparation.
Decision Guide: Coating vs. Bare Alloy
Should you coat your marine brass fitting? This table can help you decide based on the application:
| Application Scenario | Recommended Protection | Reason |
|---|---|---|
| Above deck, exposed to salt spray (e.g., rail fittings, cleats) | Marine-grade alloy + Coating | Constant salt spray and UV exposure demand maximum barrier protection. |
| Submerged seawater piping | High-grade alloy (e.g., Aluminum Brass) alone | A good coating is hard to maintain internally; the alloy must do the work. |
| Engine cooling system | Marine-grade alloy + possible internal coating | Warm, fast-moving water is aggressive; a coating adds insurance. |
| Coastal building exterior | Marine-grade alloy + Protective coating | Protects against constant salty, humid air and weather. |
The key is that a coating is part of a system, not a replacement for a poor alloy. Always start with a marine-grade brass.
What Maintenance Practices Extend the Life of Brass in Coastal Applications?
Even the best materials need care in a harsh environment. Simple, regular maintenance can double or triple the life of your brass fittings.
Regular maintenance practices that extend brass life include frequent rinsing with fresh water to remove salt deposits, applying a protective wax or oil film, conducting visual inspections for early corrosion signs, and promptly replacing any sacrificial anodes on the connected system. Consistency is far more important than intensity.

The Essential Routine: Rinse and Protect
Salt is the enemy. The single most effective thing you can do is to remove it regularly.
- Freshwater Rinse: After every exposure to saltwater—whether from a voyage, a storm, or high waves—rinse all brass fittings thoroughly with fresh, clean water. Use a hose if possible. This simple act washes away the salt before it has time to start the corrosion process. Pay special attention to crevices and joints where salt can hide.
- Dry Thoroughly: After rinsing, wipe fittings down with a soft cloth to remove standing water. Moisture left on the surface will continue to promote corrosion.
- Apply a Protective Film: Once dry, apply a thin layer of a marine-grade protectant. This could be:
- Carnauba-based paste wax: Provides a excellent water-beading barrier.
- Light machine oil or corrosion inhibitor: (like WD-40 Specialist Corrosion Inhibitor). This leaves an oily film that displaces water.
- Specialized metal protectants: Products like Boeshield T-9 are designed for this exact purpose.
Re-apply this protective film every 1-2 months, or more often in heavy use.
Inspection and Proactive System Care
Maintenance isn’t just cleaning; it’s also watching and listening to your equipment.
- Scheduled Visual Inspections: Every few months, take a close look at your brass fittings. Use the guide from the dezincification section: look for color changes, chalky residue, or pitting. Catching corrosion early lets you address it before a fitting fails.
- Check the Sacrificial Anodes: Many marine systems (like engines, hulls, or piping systems) use sacrificial zinc anodes. These blocks of zinc are designed to corrode instead of your brass and other metals. You must check these anodes regularly (at least every 6 months) and replace them when they are about 50% consumed. If the anode is gone, your brass becomes the next target.
- Avoid Galvanic Coupling: Never directly connect brass to a less noble metal like aluminum or plain steel in seawater. This will cause extremely rapid corrosion of the other metal and can also damage the brass. Use dielectric unions or insulating gaskets to separate dissimilar metals.
A Simple Annual Maintenance Checklist
Here is a basic plan to follow:
| Task | Frequency | Why It Works |
|---|---|---|
| Freshwater rinse & dry | After each saltwater exposure | Physically removes the corrosive salt electrolyte. |
| Apply protective wax/oil | Every 1-2 months | Creates a persistent barrier against salt and moisture. |
| Detailed visual inspection | Every 3-4 months | Allows for early detection and intervention on problems. |
| Check/Replace zinc anodes | Every 6-12 months | Ensures the sacrificial protection system is active. |
This routine doesn’t take much time, but it makes a huge difference. It turns a passive piece of hardware into a managed asset.
Заключение
Brass can succeed in marine environments with the right alloy, optional coatings, and consistent care. For reliable performance, choose IFAN’s marine-grade brass elbows, made from certified alloys like C46400 for proven saltwater resistance.














Последние комментарии