I once had a client face a costly system failure due to corroded brass valves. This experience taught me the critical importance of specifying the right alloy, making the question of dezincification resistance more than just academic.
Yes, the 407 brass alloy (also known as CCA407 or CZ132) is specifically engineered to resist dezincification corrosion. It achieves this through a controlled chemical composition that minimizes the selective leaching of zinc, which is the root cause of this common and destructive failure mode in standard brass fittings.
Understanding this property is vital for anyone selecting components for potable water, heating, or marine applications. Let’s break down exactly how CCA407 offers this protection and what you need to know.
What is Dezincification and Why is it a Critical Failure Mode for Brass?
Finding a brass fitting that has turned weak, porous, and pinkish-red is a sure sign of trouble. I’ve seen it lead to sudden leaks and system failures.
Dezincification is a corrosive process where zinc is selectively leached out from brass (an alloy of copper and zinc), leaving behind a weak, porous, and copper-rich structure. It is a critical failure mode because it can cause fittings and valves to fail catastrophically without obvious wall thinning, often leading to sudden leaks and flooding.
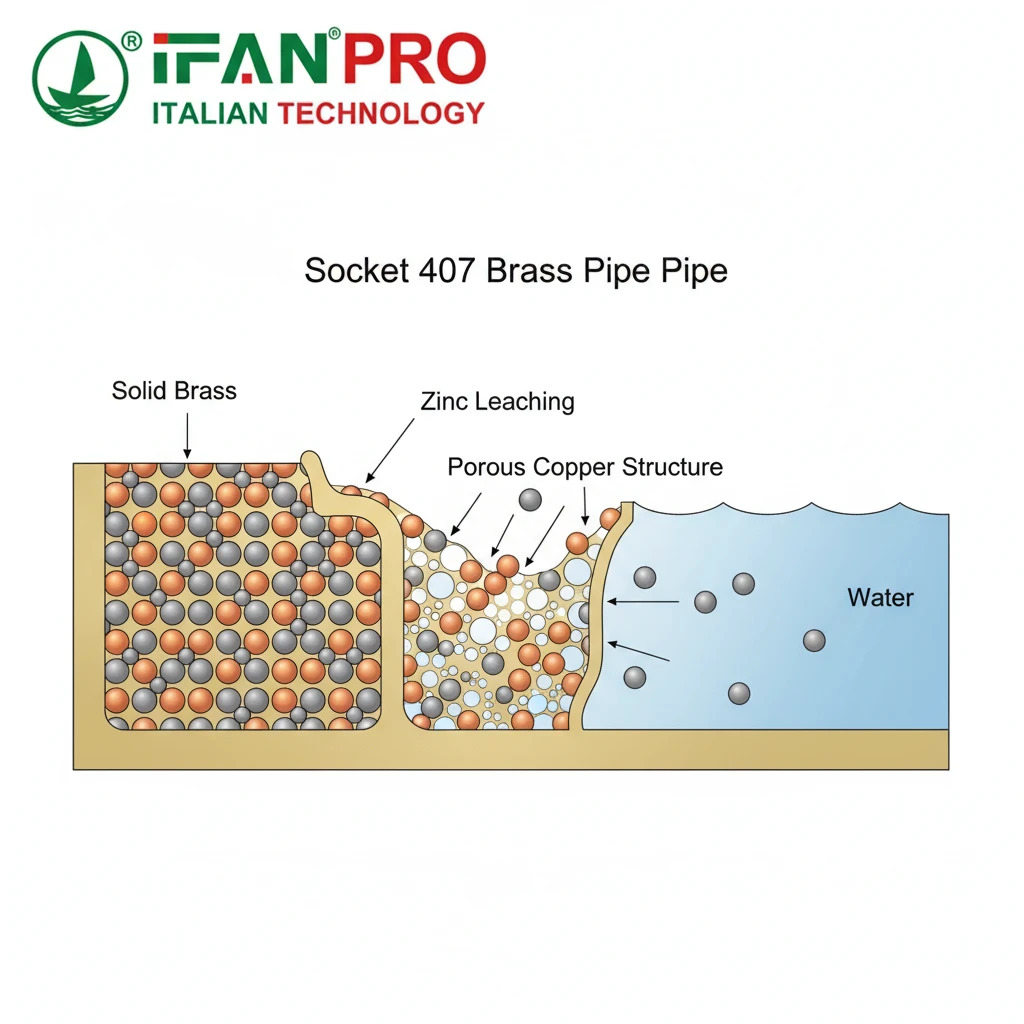
The Chemistry of a Silent Failure
To understand why dezincification is so problematic, you must first picture what brass is. Think of standard brass as a strong, uniform metal made by mixing copper and zinc together. In fact, dezincification is like a thief secretly stealing only the zinc from this mixture.
This theft happens in certain water conditions. Specifically, the zinc in the brass reacts electrochemically and dissolves into the water. Consequently, what remains is not solid brass, but a spongy, crumbly material that is mostly copper. Moreover, this leftover material loses almost all its mechanical strength. Typically, it looks like a pinkish or red, chalky residue.
Why It’s So Dangerous
The danger of dezincification lies in its stealth. Unlike general corrosion that evenly eats away at a pipe’s wall, dezincification often happens locally (plug-type) or in layers. From the outside, the fitting might look perfectly intact. However, inside, its structure becomes weak and porous. This situation can lead to a sudden, unexpected failure under normal water pressure. Therefore, you don’t get a slow drip as a warning; instead, you can get a major burst.
This makes it a critical concern for several key systems. First, Potable Water Systems face the risk of significant property damage from failure. Second, Fire Sprinkler Systems require non-negotiable reliability here. Additionally, Marine Applications see the process accelerate dramatically due to saltwater. Finally, Heating Systems may experience increased risk from higher temperatures.
The Practical Consequences
For an engineer or procurement manager, specifying a brass alloy prone to dezincification creates a major liability. Essentially, it introduces a predictable point of failure into a system. Meanwhile, for a homeowner, it can mean unexpected and very expensive repair bills. Therefore, choosing a dezincification-resistant (DZR) brass like CCA407 is not just an upgrade—it’s an essential requirement for long-term reliability and safety in most modern water systems.
How Does the Chemical Composition of CCA407 Specifically Inhibit Dezincification?
Not all brass is created equal. The secret to CCA407’s resistance is a precise recipe that stabilizes the alloy’s structure.
The chemical composition of CCA407 brass inhibits dezincification primarily through the careful addition of a small amount of arsenic (As), typically between 0.02-0.15%. This arsenic acts as an inhibitor, forming a stable protective layer on the brass surface that prevents the zinc atoms from dissolving into the water, while maintaining the alloy’s machinability and strength.
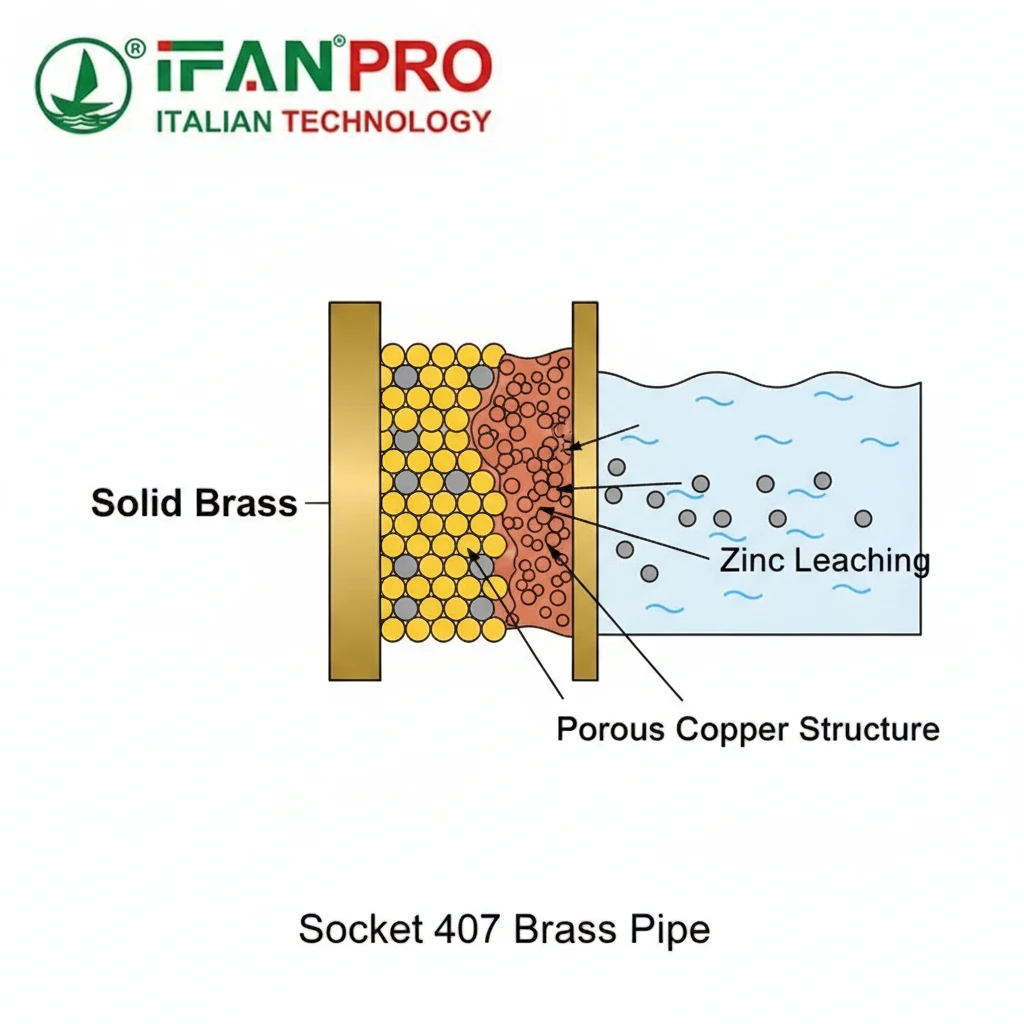
The Role of the Key Inhibitor
The standard brass alloy (like CZ121) is mainly copper and zinc, which is susceptible to dezincification. To make CCA407 resistant, manufacturers add a very small but critical element: arsenic.
Importantly, this arsenic is not free or toxic in the final product. Instead, it is metallurgically bound within the alloy structure. Essentially, its job is not to poison the water, but to poison the corrosion reaction itself. The arsenic atoms migrate to the surface of the brass when in contact with water. There, they help form a tight, stable, and adherent protective film, primarily of copper oxide. As a result, this film acts as a barrier, dramatically slowing down the electrochemical reaction that would otherwise leach zinc out of the alloy.
Balancing the Alloy for Performance
Engineers carefully balance the composition of CCA407. While arsenic is the star for corrosion resistance, they also control the amounts of copper and zinc to ensure the final product has the right physical properties.
- Copper Content: Around 58-60%. This provides the base corrosion resistance inherent to copper and ensures good formability.
- Zinc Content: Around 38-40%. This provides strength and lowers cost compared to pure copper.
- Arsenic Content: 0.02-0.15% (typical). This is the key dezincification inhibitor.
- Other Elements: Very low levels of lead (<0.09% in lead-free versions) or other elements may be present for specific machining characteristics.
A Comparison with Other Brass Alloys
This specific formulation is what sets DZR brass apart. Let’s compare it to other common types:
| Alloy Type | Common Name | Key Composition | Dezincification Resistance | Primary Use |
|---|---|---|---|---|
| CCA407 (CZ132) | DZR Brass | CuZn39Pb0.5 + As | Excellent (Engineered for it) | Critical water fittings, valves, marine hardware |
| CZ121 | Standard Brass | CuZn38Pb1.5 | Poor (Prone to it) | Decorative items, non-critical mechanical parts |
| CW617N | Leaded Brass | CuZn39Pb2 | Moderate to Poor | General plumbing fittings (being phased out) |
| Copper | – | 99.9% Cu | Immune (No zinc to leach) | Plumbing pipes, heat exchangers |
By choosing CCA407, you select an alloy whose entire composition aims for one goal: to remain stable and strong in demanding water environments. Ultimately, it prevents the silent thief of dezincification from causing a failure.
Is CCA407 Brass Certified to Relevant Dezincification Resistance Standards?
In global trade, certifications are your guarantee. At IFAN, we always ensure our brass products meet the strictest international benchmarks for performance.
Yes, CCA407 brass is widely certified to major international standards that define and test for dezincification resistance. The most important certifications include the European EN 12164 standard (for the material itself) and performance standards like EN 1254-2 for fittings, which require passing severe dezincification tests such as ISO 6509.

Two Levels of Certification: Material and Product
When we talk about certification, it happens at two levels: the material and the finished product. Reputable manufacturers and suppliers like IFAN ensure compliance at both stages.
First, the raw brass alloy itself (the rods or billets) must meet a material standard. For CCA407, the key European material standard is EN 12164:2016. This standard specifies the exact chemical composition limits, including the required arsenic content, and the mechanical properties the alloy must have. Thus, meeting EN 12164 provides the foundational proof that you are using genuine DZR brass.
The Rigorous Testing Process
However, having the right material is only the first step. Next, the finished fittings must pass tests to prove they perform as required. This is where product standards come in. For plumbing fittings, EN 1254-2 is a crucial standard. Specifically, it states that fittings made from copper and copper alloys must undergo a corrosion resistance test.
The definitive test for dezincification is ISO 6509. Laboratories use this accelerated test. They expose samples of the brass to a specific chemical solution (often containing copper chloride) for a fixed period, which simulates years of service in aggressive water. After the test, technicians examine the samples under a microscope. Then, they measure the depth of dezincification. To pass and earn the “Dezincification Resistant” (DZR) classification, the depth of corrosion must not exceed a very small limit, typically 100 micrometers.
A Guide to Global Certification Marks
Beyond the core European standards, various national certifications also validate DZR performance. A reputable CCA407 product will often carry marks from independent testing bodies. Here are the most important ones to look for:
| Certification Body | Standard / Mark | What It Signifies for CCA407 |
|---|---|---|
| European Norm (EN) | EN 12164 (Material), EN 1254-2 (Fittings) | Confirms the alloy composition and that finished fittings have passed mandated corrosion tests. |
| WRAS (UK) | Water Regulations Advisory Scheme | Approves products for contact with drinking water in the UK, requiring proof of DZR resistance. |
| ACS (France) | Attestation de Conformité Sanitaire | French certification for drinking water safety, which includes stringent material testing. |
| KIWA (Netherlands) | KIWA Watermark | A leading European quality mark for construction products in drinking water systems. |
| NSF/ANSI 61 (USA) | NSF International | Certifies the product does not leach contaminants into drinking water; specific sections address alloy compliance. |
When you source CCA407 brass fittings, always request documentation that proves compliance with these standards. Ultimately, this documentation gives you assurance that the product has not only been made from the right recipe but has also passed the practical exam for long-term durability in your specific application.
What Water Chemistry Conditions Still Pose a Risk to 407 Brass Alloy?
Even the best armor has its limits. I always advise clients that understanding water conditions is essential to maximizing the lifespan of any brass component, including CCA407.
While highly resistant, CCA407 brass can still be at risk under extreme or sustained adverse water chemistry conditions. The primary risks come from very low pH (acidic water), very high chloride content (like in seawater or heavy softening), high temperatures, and the presence of stagnant or low-flow water which allows aggressive conditions to develop at the metal surface.
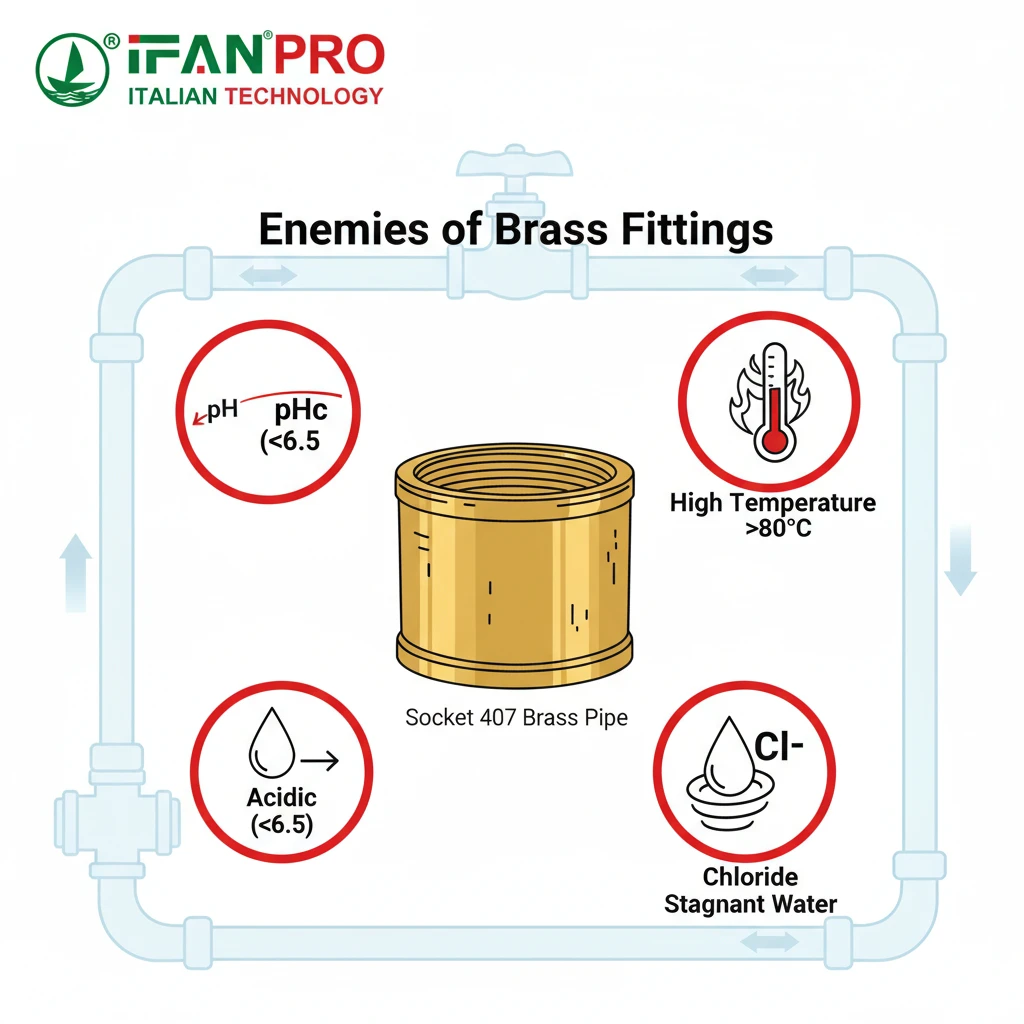
Understanding the Threat of Acidic Water
CCA407 is engineered for excellent performance in normal potable water. However, “normal” can vary widely. First, let’s consider low pH or acidic water. Water with a pH below 6.5 is considered acidic. This acidity aggressively attacks the protective oxide layer that forms on brass. If this layer constantly dissolves faster than it can re-form, the underlying alloy becomes vulnerable. Consequently, this process can eventually compromise the dezincification inhibition. For instance, if your water source is soft and naturally acidic, or if pH correction is not managed in a closed system, it increases the long-term risk. Therefore, you should always test your water’s pH first.
The Challenge of High Chloride Content
Another major concern is high chloride and sulfate content. Chlorides are highly corrosive ions. Typically, you find them in high concentrations in seawater, brackish water, and in water softened by certain salt-based systems. They can penetrate the protective surface layer on the brass. Although CCA407 performs much better than standard brass, extremely high chloride levels combined with other factors (like low flow) can accelerate corrosion over many years. For example, marine environments demand extra caution and possibly a different material altogether.
How Temperature and Stagnation Create Risk
Next, high temperature presents a significant risk. As a general rule, chemical reactions double in speed with every 10°C (18°F) increase in temperature. This rule applies to corrosion too. CCA407 in a domestic hot water line at 60-80°C will face a more aggressive environment than in a cold water line. Although the alloy is rated for these temperatures, its lifespan in very hot systems (like those above 80°C) may be shorter compared to cooler applications.
Finally, stagnant or low-flow conditions are often overlooked. Essentially, flowing water helps maintain uniform water chemistry and temperature at the pipe wall. In contrast, stagnant water (like in a seldom-used branch line, a dead leg, or a system that is periodically shut down) allows oxygen to deplete. Furthermore, local concentrations of chlorides or other salts can become very high. This situation creates localized “cells” of highly aggressive water that can attack the brass, potentially leading to localized pitting or crevice corrosion, even if the overall water quality is good. Consequently, you should design systems to minimize dead legs whenever possible.
How to Assess Your Specific Risk
Use this table to assess the environment for your CCA407 brass components:
| Water Condition | Low Risk | Medium Risk (Monitor/Consider) | High Risk (Consult an Expert) |
|---|---|---|---|
| pH Level | 7.0 – 8.5 | 6.5 – 7.0 or 8.5 – 9.0 | Below 6.5 or above 9.0 |
| Chloride Content | < 100 mg/L | 100 – 250 mg/L | > 250 mg/L (or seawater) |
| Water Temperature | < 60°C (140°F) | 60°C – 80°C (140°F – 176°F) | > 80°C (176°F) |
| Flow Conditions | Constant, turbulent flow | Intermittent use | Stagnant, dead legs present |
Final Recommendations and Material Alternatives
For high-risk applications (e.g., marine, industrial process water), you may need a material upgrade. More resistant alloys like DZP (Dezincification Proof) brass with even higher copper content, naval brass, or even bronze could be necessary. Always conduct a water analysis for critical systems. Meanwhile, for standard potable water, correctly specified CCA407 offers outstanding and reliable performance. As a result, knowing your water is the key to making the best and safest choice for your specific application.
Conclusion
CCA407 brass effectively resists dezincification, making it a reliable choice for most water systems. For guaranteed, certified CCA407 brass fittings and valves, trust IFAN as your supply chain partner.














Commentaires récents