A client once insisted on the bright look of chrome but needed the reliability of brass. This request is common, and the answer is a definitive yes.
Yes, brass elbow fittings can be chrome-plated for excellent aesthetic results. Brass is actually one of the best base metals for chrome plating because it is smooth, dense, and bonds well with plating layers. The process creates a brilliant, mirror-like finish that enhances the visual appeal of plumbing fixtures, door handles, and decorative hardware.
While the result is beautiful, the journey from raw brass to shiny chrome involves critical steps. Let’s explore the process, benefits, and key considerations to ensure a flawless finish.
What is the Standard Process for Electroplating Chrome onto Brass Fittings?
The shine you see is the result of a meticulous, multi-stage process. Rushing any step leads to a poor finish.
The standard electroplating process involves cleaning and preparing the brass surface, then sequentially plating layers of copper, nickel, and finally chrome in an electrochemical bath. This multi-layer approach ensures adhesion, smoothness, and the final brilliant, durable chrome appearance.
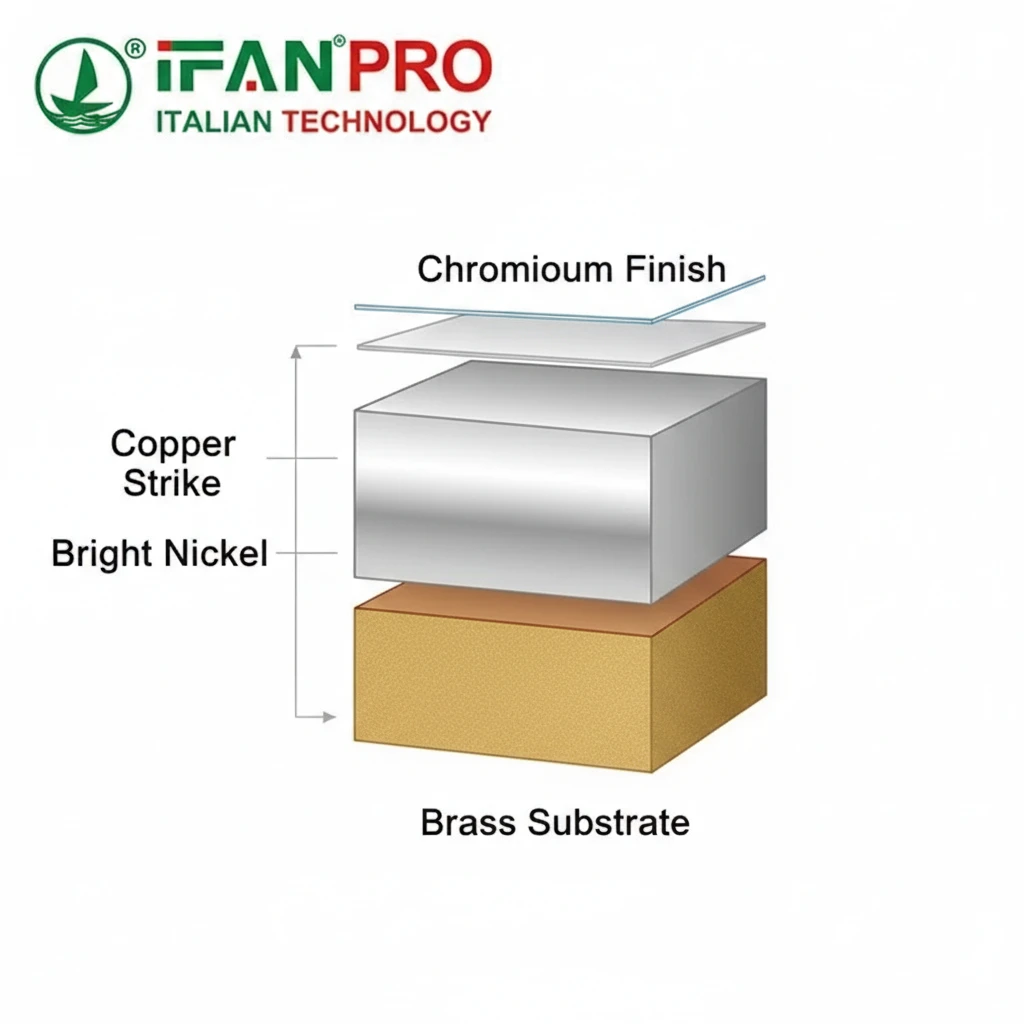
Step-by-Step Journey to a Mirror Finish
The transformation of a brass fitting into a chrome-plated masterpiece is not a single step but a series of precise stages. Each stage has a specific purpose.
Step 1: Cleaning and Preparation. This is the most critical phase. Any oil, dirt, or oxidation on the brass surface will prevent proper adhesion. The fittings undergo alkaline cleaning, acid dipping, and electrocleaning to achieve a perfectly pristine surface.
Step 2: Copper Plating. The clean brass parts receive a thin layer of copper. Copper plating offers two main benefits. First, it provides an excellent base for the subsequent nickel layer, ensuring strong adhesion. Second, it helps to fill in tiny imperfections on the brass surface, creating a smoother foundation.
Step 3: Nickel Plating. This is the workhorse layer. A thick layer of nickel deposits onto the copper. Nickel provides the real corrosion resistance and the bulk of the coating’s durability. The plating bath often includes brightening agents to give the nickel a very smooth, reflective surface, which is crucial for the final chrome look. Sometimes, a semi-bright nickel layer goes down first, followed by a bright nickel layer for maximum protection and shine.
Step 4: Chrome Plating. The final step is a very thin layer of chromium. Surprisingly, the classic chrome finish is only about 0.3 to 1.0 microns thick—thinner than a human hair. This thin layer is porous but provides a hard, wear-resistant top coat that brings the brilliant blue-white shine and protects the nickel beneath from tarnishing.
Visualizing the Electroplating Layers
The table below summarizes the role of each layer in the finished product:
| Plating Layer | Primary Function | Key Characteristic |
|---|---|---|
| Copper | Adhesion & Smoothing | Bonds to brass and fills microscopic pores. |
| Nickel | Corrosion Resistance & Bulk | Provides thickness, durability, and underlying brightness. |
| Chromium | Final Hardness & Aesthetic Shine | Adds a thin, hard, blue-white mirror finish. |
The quality of the final product depends entirely on the control of each bath’s chemistry, temperature, current, and timing. A reputable plater follows strict standards at every stage.
How Does Chrome Plating Affect the Corrosion Resistance of the Brass Elbow?
Chrome plating is not just for looks; it fundamentally changes how the fitting withstands the environment.
Chrome plating significantly improves the corrosion resistance of a brass elbow. The underlying nickel layer acts as a robust, non-porous barrier against moisture and chemicals, while the top chrome layer provides a hard, inert shell. Together, they protect the base brass from tarnishing, oxidation, and dezincification (a form of corrosion specific to brass).

Creating a Protective Shield
Brass alone has moderate corrosion resistance, but it can tarnish and, in certain water conditions, corrode. Chrome plating builds a superior defensive system.
The real hero is the nickel layer. Nickel is much more resistant to corrosion than brass. It forms a continuous, impermeable barrier that blocks water, oxygen, and salts from ever reaching the brass substrate. This prevents the primary forms of brass corrosion.
The top chrome layer seals the deal. While thin and micro-porous, it is extremely hard and chemically inert. It protects the nickel from surface scratches and minor abrasions that could expose it to the elements. The classic “chrome” look is, in fact, the look of the bright nickel layer shining through the clear chromium top coat.
Understanding Potential Failure Modes
However, the plating must be high-quality and applied correctly. If it is too thin, applied over a dirty surface, or damaged during installation, corrosion can start.
The most common failure is “pitting corrosion.” If a scratch goes deep enough to penetrate both the chrome and nickel layers, exposing the brass, an electrochemical cell can form. Moisture triggers corrosion at this small point, which can spread underneath the plating, causing it to blister and flake off.
Another risk is poor coverage on sharp edges or internal threads, where plating thickness can be minimal. This can become a starting point for rust or tarnish.
Corrosion Protection Comparison
The table below shows how plating improves performance:
| Fitting Type | Resistance to Tarnishing | Resistance to Salt Spray/Weathering | Resistance to Dezincification |
|---|---|---|---|
| Raw Brass | Poor (will darken) | Fair | Poor (in soft/acidic water) |
| Decorative Chrome-Plated Brass | Excellent | Very Good | Excellent (brass is fully sealed) |
| Industrial Hard Chrome | Excellent | Excellent | Excellent |
For most indoor aesthetic applications and standard outdoor use, a properly applied decorative chrome plating provides outstanding and long-lasting corrosion protection for the brass fitting underneath.
Is the Plating Durable Enough for High-Touch or Frequently Cleaned Areas?
Doorknobs, lever handles, and visible plumbing fittings get constant use. The plating must withstand this daily abuse.
Yes, quality chrome plating is exceptionally durable for high-touch areas. The chromium top layer is very hard and scratch-resistant, while the thick nickel underlayer provides cushioning and adhesion. This combination withstands frequent handling, cleaning with common household products, and resists wear far better than raw brass or painted finishes.

The Science of Surface Hardness
Durability in plating is measured by hardness, adhesion, and thickness. Chrome plating excels in these areas.
The Vickers hardness of chrome plating is very high—around 800-1000 HV. For comparison, raw brass is about 150 HV. This means the chrome surface is highly resistant to scratches from keys, rings, or abrasive dust. It won’t easily mar from normal contact.
The nickel underlayer is softer but tough. It acts as a flexible buffer that absorbs impact and prevents the hard, brittle chrome layer from cracking under pressure. The strong bond between the copper, nickel, and chrome layers (achieved through proper preparation) is what prevents the plating from peeling or flaking when the fitting is twisted or impacted during installation or use.
Performance with Cleaning and Chemicals
Frequently cleaned areas, like bathroom or kitchen fixtures, face chemical wear. Quality chrome plating performs well here.
It is highly resistant to:
- Water and soaps
- Most common household cleaners (non-abrasive)
- Alcohol-based solutions
- Mild acids and oils
However, you must avoid:
- Abrasive cleaners (scouring powders, steel wool): These will scratch any surface, including chrome, dulling its shine over time.
- Strong acidic cleaners (like those for heavy lime scale): These can attack the micro-porous chrome layer over prolonged, repeated exposure.
For maintenance, a soft cloth with mild soap and water is ideal. This routine cleaning will keep the fixture looking new for years without damaging the plating.
Testing Plating Durability
Manufacturers use standard tests to ensure durability. Two key tests are:
- Salt Spray Test (ASTM B117): Fittings sit in a salt fog chamber for hours to simulate years of corrosive exposure. Good plating shows no base metal corrosion.
- Adhesion Test (Tape Test): A cross-hatch pattern is cut into the plating, and strong tape is applied and ripped off. Good plating shows no flaking or removal.
The table below summarizes durability in different scenarios:
| Use Case | Durability of Quality Chrome Plating | Maintenance Tip |
|---|---|---|
| Door Handles / Knobs | Excellent. Withstands millions of touches. | Clean with damp cloth to remove hand oils. |
| Faucet Handles | Excellent. Resists water and frequent turning. | Avoid harsh chemicals; dry after cleaning. |
| Decorative Plumbing Fittings | Excellent. Stable in humid environments. | Use vinegar solution for scale; rinse thoroughly. |
| Exterior Hardware | Very Good. Protects against weather, but may require more frequent cleaning. | Rinse off road salt or pollutants. |
In short, for nearly all residential and commercial interior applications, chrome plating offers an ideal balance of lasting beauty and practical durability.
Are There Specific Brass Alloys That Accept Chrome Plating Better Than Others?
The base metal matters. Not all brass is created equal for plating.
Yes, brass alloys with higher copper content and controlled impurities accept chrome plating better. Alloys like C26000 (Cartridge Brass, 70% Cu, 30% Zn) are excellent choices. They have a smooth, uniform microstructure that allows for even etching and plating, resulting in a brighter, more adherent, and more consistent final finish.

Why Alloy Composition is Critical
The plating process involves a chemical and electrochemical “conversation” with the base metal. The alloy’s composition determines how smooth that conversation goes.
Ideal Alloys (High Copper, Lead-Free):
- C26000 (Cartridge Brass): This is the gold standard for plating. Its 70/30 copper-zinc ratio offers excellent ductility and a very uniform grain structure. When the plater etches (lightly acids) the surface before plating, it attacks evenly, creating a perfect bonding surface. The result is a brilliant, flawless finish.
- C27200 (Yellow Brass, 65% Cu): Another very good option. Slightly less copper but still provides excellent plating characteristics for most applications.
Alloys with Challenges:
- High-Zinc Brasses (e.g., C28000, Muntz Metal): These have 60% copper or less. They are more prone to a defect called “staining” or “cloudiness” after plating. The zinc can react differently during etching, leading to a less uniform surface and a final finish that might lack maximum brilliance.
- Leaded Brasses (e.g., C36000): These are easy to machine but problematic for plating. Lead particles in the alloy do not plate. They can cause “skip plating” (bare spots) or lead to blistering as the plating tries to cover an inconsistent surface. Most high-quality platers will refuse to plate leaded brass for decorative chrome.
The Role of Surface Finish and Porosity
Even with a good alloy, the initial surface condition of the brass fitting is vital. The brass must be:
- Smooth: Free of deep tool marks or pits, which can trap plating solutions and cause bleeding or defects.
- Non-Porous: Cast brass fittings can sometimes have micro-porosity, which traps chemicals and causes later blistering. Wrought or forged brass (like that used in IFAN fittings) is denser and superior for plating.
Choosing the Right Brass for the Job
The table below provides a quick guide:
| Brass Alloy | Typical Composition | Plating Suitability | Common Use |
|---|---|---|---|
| C26000 (Cartridge Brass) | 70% Cu, 30% Zn | Excellent. Preferred for high-quality decorative plating. | Premium plumbing fittings, decorative hardware. |
| C27200 (Yellow Brass) | 65% Cu, 35% Zn | Very Good. Reliable for most applications. | Standard plumbing fittings, connectors. |
| C36000 (Free-Cutting Brass) | 61.5% Cu, 35.5% Zn, 3% Pb | Poor. Not recommended for decorative chrome. Lead causes defects. | Machined components where plating is not needed. |
| C28000 (Muntz Metal) | 60% Cu, 40% Zn | Fair. Can be plated but may not achieve premium brightness. | Some industrial applications. |
For the best aesthetic results, always specify fittings made from high-copper, lead-free brass alloys like C26000. This ensures the plater can deliver the mirror finish you expect.
Conclusion
Yes, brass elbows can be beautifully and durably chrome-plated for aesthetics. For guaranteed quality, choose IFAN’s premium brass fittings, made from plating-grade alloys and finished to the highest standards.














Commentaires récents